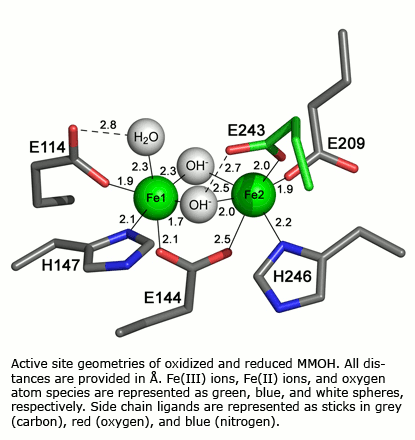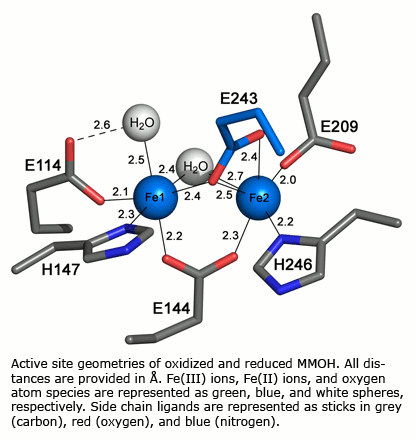
Polymetallic centers occur in numerous metalloproteins where they perform a variety of functions including hydroxylation of methane, the generation of amino acid radicals, hydrolysis of phosphate esters, and dioxygen transport. How do two or more metal atoms in close proximity in these proteins work in concert to carry out their biological function? How do we know the coordination environment of such polymetallic species in biological molecules? To help answer these questions, we synthesize and characterize well-defined model compounds and compare their properties with those of their biological counterparts. Physical and biological studies of the proteins are also undertaken. Recently our attention has focused on systems in which one or more carboxylate groups link two metal ions. Proteins in this category include hemerythrin, the dioxygen transport protein of marine invertebrates, methane monooxygenase, an amazing biomolecular machine that converts methane and dioxygen selectively to methanol and water, and the related systems toluene/o-xylene and phenol monooxygenase. We use chemical, EPR, Raman and optical spectroscopic, redox, EXAFS, X-ray crystallographic, NMR, Mössbauer and magnetic, and freeze-quench and stopped flow kinetics techniques to determine the effects of one or more metals on the properties of an adjacent metal in both the model compounds and proteins. Under investigation is the dioxygen reactivity of reduced forms of dinuclear iron complexes and proteins by time-resolved UV/visible, EPR, Mössbauer, and resonance Raman laser spectroscopy. Intermediates are also being probed by low temperature X-ray crystallography. Details of reaction intermediates and transition states are also studies by density functional theoretical studies in collaboration with the Friesner laboratory at Columbia University.
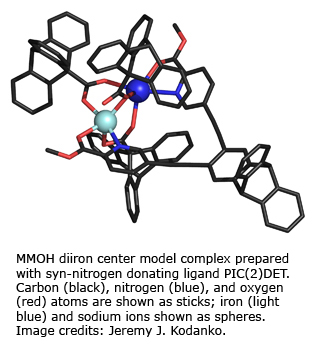
Studies with synthetic models focus on the use of sterically hindered carboxylate and preorganized ligands to afford complexes that best mimic the stoichiometric and functional properties of the enzyme active sites. With the use of the models we now have carboxylate-bridged diiron systems that can perform C–H bond activation and oxo-transfer reactions.
Both physical and mechanistic studies of soluble methane monooxygenase, toluene/o-xylene monooxygenase, and phenol hydroxylase are currently in progress to characterize the active site geometries and the catalytic mechanism of the hydroxylase components (MMOH, ToMOH, PHH) of these fascinating enzymes. Genetic studies of the native sMMO operon and of the ToMO and PH proteins expressed in E. coli, mechanistic investigations of the MMOH, ToMOH, and PHH catalytic reaction cycles, spectroscopic and X-ray crystallographic analyses of key intermediates in the reactions, and details of the hydrocarbon activation chemistry are in progress. Also being investigated are the structures and functions of the reductases, coupling, and other proteins in the sMMO, ToMO, and PH systems alone and in complexes with the other components.
Solution NMR spectroscopy has been used to determine the structures of MMOB, the coupling protein, and the ferredoxin and flavin components of MMOR. 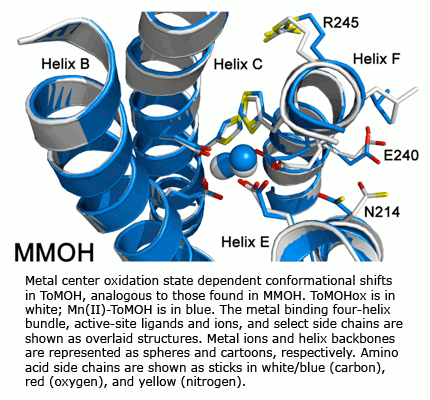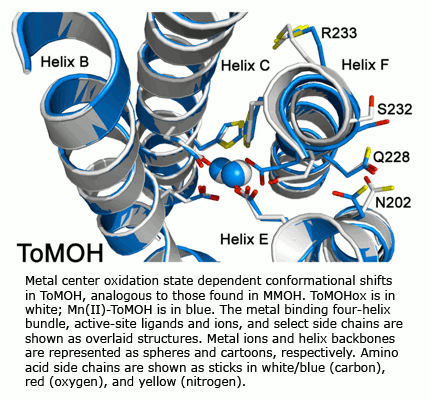 The electron transfer reactions of MMOR are also being studied. Recently, expression in the native organism of a fourth protein in the sMMO operon has been demonstrated. The properties and function of this protein, MMOD, are under investigation. Additional proteins in the system have also been identified and are being characterized. Recent X-ray crystal structure determinations of ToMOH and PHH have revealed important features about substrate access to their catalytic diiron centers that guide site-directed mutagenesis work to probe features of their O2 activation and hydrocarbon oxidation chemistry.
The electron transfer reactions of MMOR are also being studied. Recently, expression in the native organism of a fourth protein in the sMMO operon has been demonstrated. The properties and function of this protein, MMOD, are under investigation. Additional proteins in the system have also been identified and are being characterized. Recent X-ray crystal structure determinations of ToMOH and PHH have revealed important features about substrate access to their catalytic diiron centers that guide site-directed mutagenesis work to probe features of their O2 activation and hydrocarbon oxidation chemistry.